

2022 年 3 月,美国食品药品监督管理局 (FDA) 发布了,针对开发 CAR-T细胞治疗的机构的指南草案。 该指南包括对 CAR-T 细胞开发的各个阶段的具体建议——包括临床前测试、生产和控制(CMC)和这些细胞药物在患者输注后长达 15 年的监测。
我国首部《CAR-T细胞治疗恶性血液肿瘤及免疫靶向治疗相关感染管理指南》中对接受CAR-T治疗患者的长期随访进行了详细推荐。
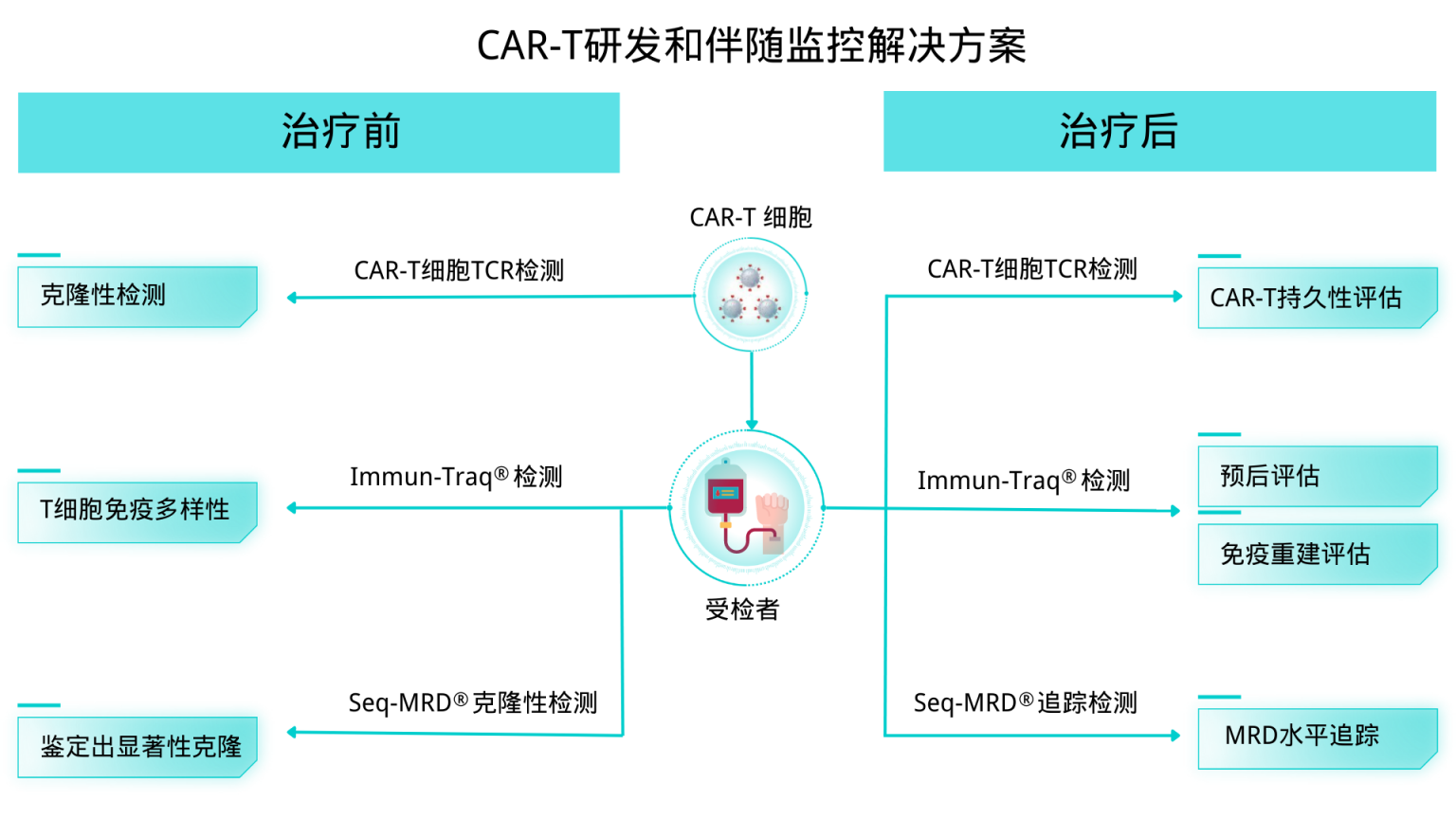
所以对于CAR-T治疗后的CAR-T持久性评估,治疗后效果评估,以及免疫重建水平的监控就非常重要。艾沐蒽结合自身产品,包括Seq-MRD®,Immun-Traq®,和ImmuHub®的技术优势,整合了一套专为CAR-T的研发和监控的伴随解决方案。
在CAR-T研发中,本解决方案可以用来评估CAR-T疗法的效果。它可以帮助研究人员评估CAR-T对免疫系统的影响,并且在治疗后的随访过程中评估免疫系统的重建情况,并评估CAR-T细胞的持久性。它可以帮助研究人员了解疗法的效果,更好地评估疗效,并有效地监控治疗后的情况。
艾沐蒽发表的相关文献:
Seq-MRD®部分参考文献:
- Yan N, Wang ZL, Wang XJ, et,al. Measurable residual disease testing by next generation sequencing is more accurate compared with multiparameter flow cytometry in adults with B-cell acute lymphoblastic leukemia. Cancer Lett. 2024 Jul 4;598:217104. doi: 10.1016/j.canlet.2024.217104.
- Min’er Gu, et al. The effectiveness of blinatumomab in clearing measurable residual disease in pediatric B-cell acute lymphoblastic leukemia patients detected by next-generation sequencing. Cancer Medicine (2023) https://doi.org/10.1002/cam4.6771
- H.Chen. et al. Minimal residual disease detection by next-generation sequencing of different immunoglobulin gene rearrangements in pediatric B-ALL. Nature Communications (2023) https://www.nature.com/articles/s41467-023-43171-9
- Huang, Y. et al. Predictive value of next-generation sequencing-based minimal residual disease after CAR-T cell therapy. BMT (2022). https://www.nature.com/articles/s41409-022-01699-2
Immun-Traq®部分参考文献:
- Yongxian Hu. et.al. Sequential CD7 CAR T-Cell Therapy and Allogeneic HSCT without GVHD Prophylaxis. The New England Journal of Medicine(2024). https://www.nejm.org/doi/pdf/10.1056/NEJMoa2313812
- Wang, X. et al. Quantitative characterization of T-cell repertoire alteration in Chinese patients with B-cell acute lymphocyte leukemia after CAR-T therapy. Bone Marrow Transplantation (2019). https://www.nature.com/articles/s41409-019-0625-y
ImmuHub®技术平台参考文献:
